GMP Lab
Within the ICT of the University Research Center of the University of Patras operates a newly established GMP Facility (Good Manufacturing Practice) with the purpose of graft manipulation, cell therapy production according to international standards and guidelines followed by the HCT program of the BMT Unit of the University General Hospital of Patras.
The facility is licensed for GMP production of cell and gene therapies by the National Medicines Agency. Prot. No.: 80551/2023 29.02.2024
The GMP Facility constists of a Gowning/Degowning room (D), two Cell Processing rooms (B & C) and a Cryopreservation room (F) that are interconnected via pass boxes.
You can take a virtual tour of our lab here.
The layouts of all GMP lab rooms as well as the layouts of the quality control rooms are found here.
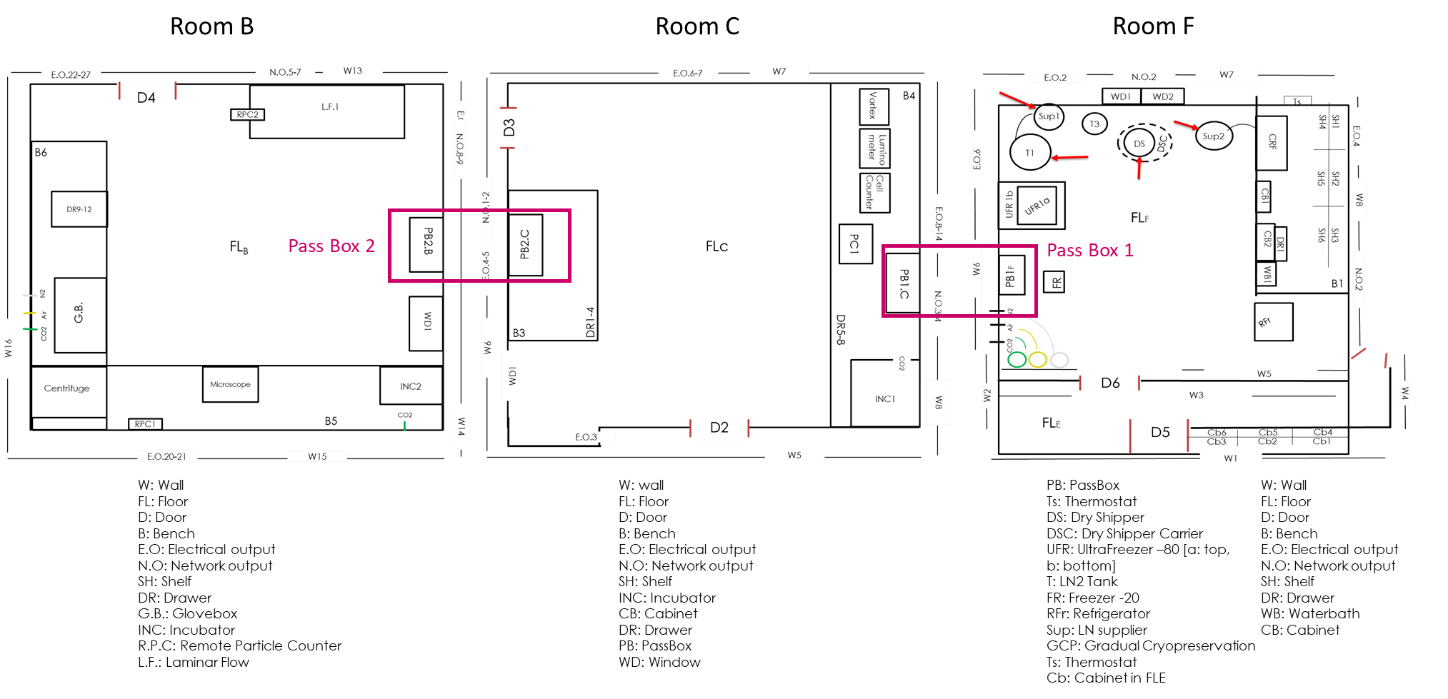 Outline of the Cell Processing rooms (B & C) and the Cryopreservation room (F) of the GMP Facility within the ICT. The coloured boxes depict the interconnections via pass boxes.
Outline of the Cell Processing rooms (B & C) and the Cryopreservation room (F) of the GMP Facility within the ICT. The coloured boxes depict the interconnections via pass boxes.
The Cell Processing rooms (B & C) are connected via an intermediate airlock space (B/C) while the entrance to the facility takes place through the gowning/degowning room (D).
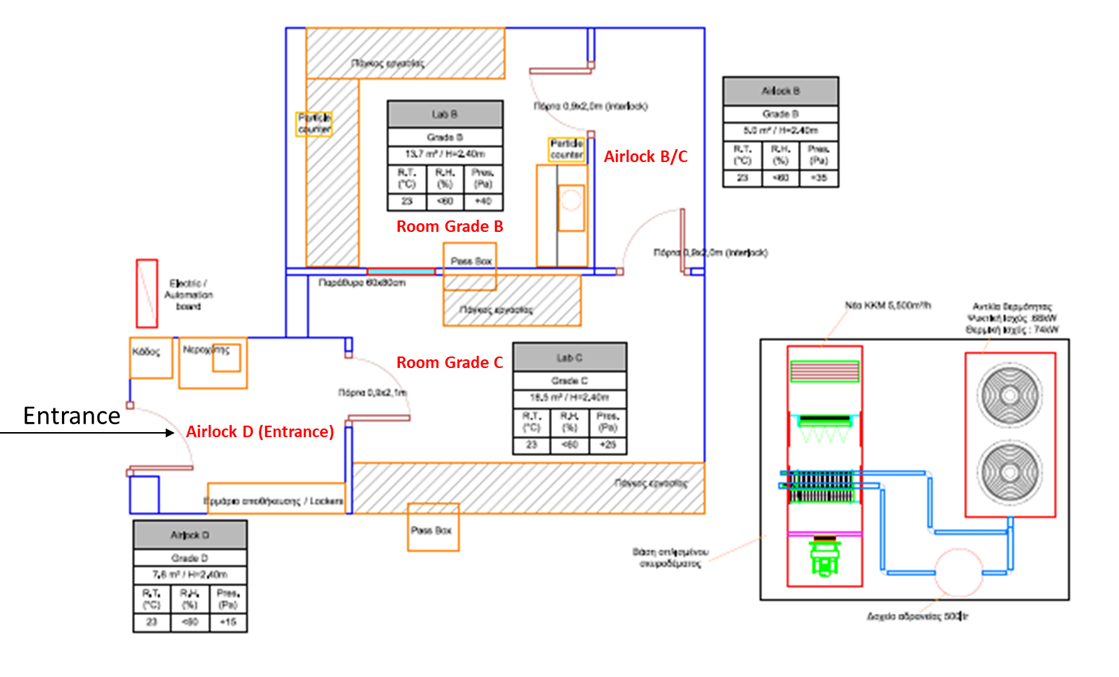
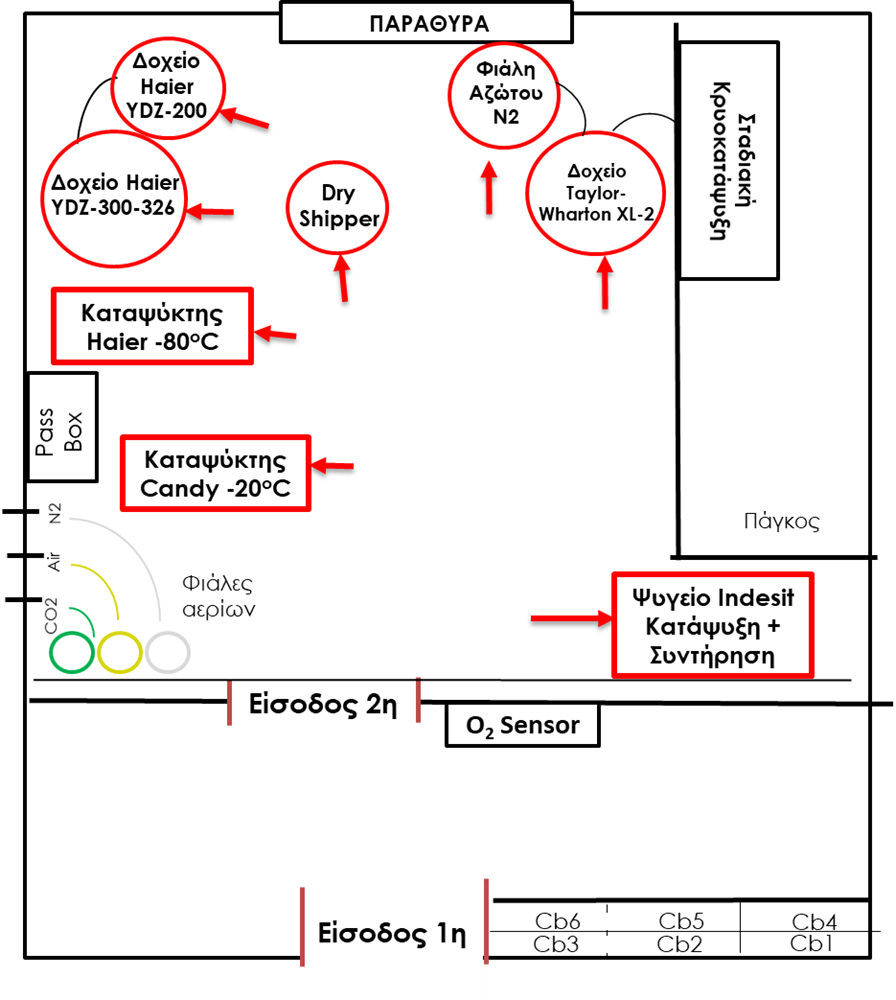
The Cryopreservation room (F) is located in parallel to the room C and is accessible through a separate entrance.
All files related with the instalatation and operational protocols can be found here.
Funding